Unit of Rate Constant for Third Order Reaction
The unit of the rate constant in a zero-order reaction is given by concentrationtime or Ms where M is the molarity and s refers to one second. The proportionality constant k is known as the rate constant and is specific for the reaction shown at a particular temperature.

Solved In A Second Order Reaction The Units Of Rate Constant Are N
M-7 time-1 O b.

. M -2 s -1 M -2 min -1 M -2 hr -1 etc. The concentrations of all three species are. M - n-1 s -1 etc.
Rt is the initial rate as a function of time t k is the rate constant A is the concentration of A and n is the order of A. The unit of rate and rate constant are same for a. The rate refers to the rate of the reaction.
What is the unit of the rate constant k for an overall third-order reaction. First Order Reactions rate kA Mt k M k units. The rate constant changes with temperature and its units depend on the sum of the concentration term exponents in the rate law.
Characteristics of third order reaction 1 Unit of K-K 1 2t x 2a-x a 2a x 2 unit of K 1 time mole litre-1 mole litre-1 mole litre-1 2 x mole litre-12. Rate k A3 rate k A2 B rate k A B C Mt k M3. Where rate is in meters per second or rather muller per second.
Rate k A2 rate k A B Mt k M2. Rate k A n. Zero Order Reactions rate kA0 Mt k k units.
Begin array lRate -frac dA dtk A 0 kend array Where. Unit of rate is given by R mol Ls mol L-1 s-1 mol L-1 s-1 k mol L-1 3 k L 2 mol-2 s-1. Rt kAn.
2 The unit of velocity constant depends upon the units of concentration because. Thus we write rate equals K. Rate k A Mt k M.
Find the rate constant unit for the reaction 2NOO 2 2NO 2 1 Mark Ans. Rate k An Mt k Mn. Rate k A 3 rate k A 2 B rate k A B C Mt k M 3.
Rate k A0 Mt k. The Differential form of a zero-order reaction can be written as. Second Order Reactions rate kA2 rate kAB Mt k M2 k unit.
5 rows In second example from the previous lesson a second-order reaction we found the units for k to. The Rate Constant of Third Order Irreversible Reaction with Two Equal Reactant Concentrations formula is defined as the proportionality constant in the equation that expresses the relationship between the rate of chemical reaction and the concentrations of the reacting substances and is represented as k r C A C B2 or Third Order Reaction Rate Constant Reaction Rate. Different Cases in 3rd Order Kinetics.
In this video you will learn the Units of rate constant for zero order first order second order third order and nth order reactionUnits of rate constant. M -1 s -1 M -1 min -1 M -1 hr -1 etc. M-2 time Clear my choice.
Is the concentration in Mueller. Rate kA 3 rate kA 2B rate kABC Mt k M 3. For a zero order reaction the rate constant has units molar per second Ms or mole per liter per second molL 1s 1 What are the units for third order reaction.
S-1 min-1 hr-1 etc. L2mol2 sec QUESTION 11 Given the data below for the reaction 2A 20-4CDE35 the reaction is order in A order in order in Cand order overall. If the units of time are s and of concentration are M then.
Here stands for the concentration or rather the order of the reaction with respect. Is the rate constant. It is defined as the rate of disappearance of reactant and the rate of.
Rate Constant k has UNITS. Times a race to the power of em. The rate constant is unmerically the same for three reactions of first second and third order respectively the unit of concentration being in moles asked Jun 7 2019 in Chemistry by KritikaChakraborty 898k points.
Since A is assumed the only reactant for simplicity its order IS the reaction order. The rate of the reaction 2NOO 2 2NO 2 is given by RatekNO 2 O 2 Since it is a third-order reaction the unit of the rate constant is mol-2 s-1. M -2s -1 M -2min -1 M -2hr -1 etc.
And the small M. Mt k M n. It depends on the order of the reaction.
Third Order Reaction Rate Constant - The Third Order Reaction Rate Constant is defined as the average rate of the reaction per concentration of the reactant having power raised to 3. The rate law can be expressed as. Measured in Square Cubic Meter per square Mole per Second Reactant A Concentration - Reactant A Concentration refers to the amount of reactant A present in the solvent at any given.
Three different cases may occur in the third-order reaction. Units of rate constant for n th order m o l l i t 1 1 n t 1 For third order reaction n 3 Units are m o l l i t 1 1 3 t 1 m o l l 2 l i t 2 t 1. Or it can also be more per minute muller are depending on what whichever unit is given for the time.
In this we have discussed about the rate rate of reaction and order of reactionsand also aboutzero order reactionzero order half Lifeand their examplespd. What is the unit of the rate constant k for an overall third-order reaction. Ms Mmin Mhr etc.
Answer 1 of 6. QUESTION 10 Which of the following would be a reasonable unit for the rate constant of a third order reaction. K is the rate constant of the reaction.
Unit of K litre 2 mole -2 time-2.

Units Of Rate Constant Zero Order First Order Second Order Third Order Nth Order Reaction Youtube
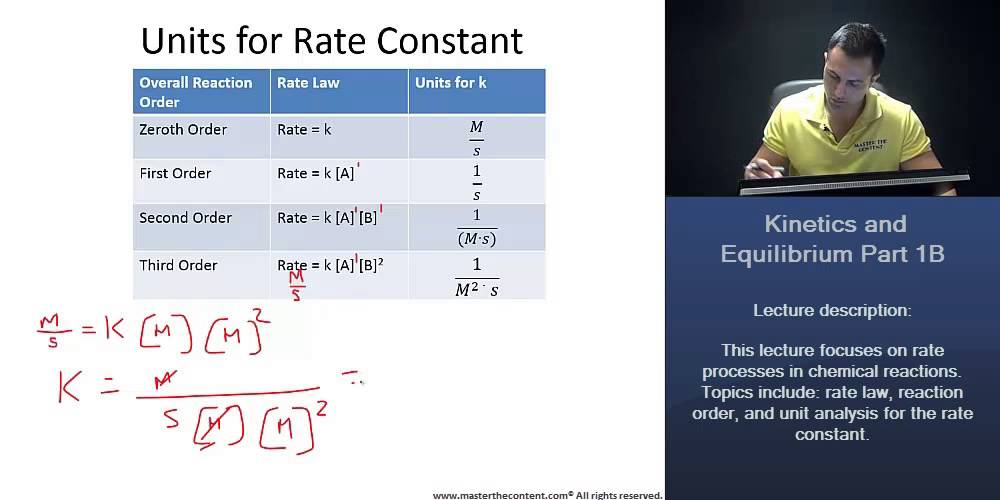
Mcat Units For Rate Constant Zeroth Order First Order Second Order Third Order Youtube
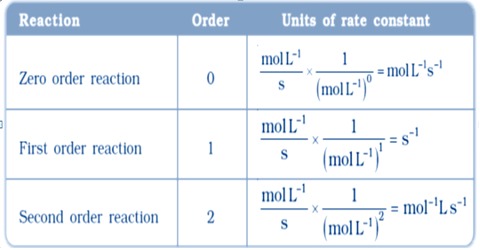
Comments
Post a Comment